In recent years we have increasingly seen conflicting expectations among consumers. Demand for ever increasing performance characteristics while minimizing or eliminating negative environmental impacts for ever-developing technical applications, specially selected materials with defined properties are required. The need for new material developments that meet both performance and environmental protection requirements leads us to further examine iron oxide.
Iron Oxide in Coating Systems
The use of nanoparticulate iron oxide can significantly improve product surface quality in coating systems, which reduces material losses during the processing of steel through scale conditioning. [1]
By reducing solid state friction (affecting tribological properties), a significant reduction in tool wear can be achieved. Additionally, reduced scale formation on forged parts can improve the quality of the product surfaces: Defects and material loss during forging are appreciably reduced.
Iron Oxide as a Battery Material
Stationary energy storage with our battery material is another area of great potential. The demand for local electrical energy storage is increasing because the increasing proportions of renewable energies in the electricity mix mean a higher need for the intermediate storage of the energy generated independently of the consumption. Cost-effective energy storage systems that are readily available, non-toxic and environmentally friendly are essential for the green revolution. Iron-air accumulators precisely meet these requirements [2], for which iron oxide nanoparticles are indispensable. [3]
Drawbacks of Previous Production Methods
The search for an effective, scalable and continuous production method for nanoscale iron oxide is of critical importance! [4,5] Previous production methods resulting in high quality material were often multi-stage processes and too complex and expensive for the emerging fields of application. Sol-gel and chemical precipitation for example have always been associated with multi-stage, complex production processes which are both energy and resource-intensive, making the processes expensive, difficult to scale and requiring the use of toxic chemicals to yield limited amounts - a large-volume manufacturing is more complicated.
Single-Stage Production in the Pulsation Reactor
IBU-tec has developed a single-stage method for the production of iron oxide nanoparticles in our pulsation reactor – a globally exclusive thermal manufacturing process, scalable from technical stage to multi ton output.
This single-stage process can reduce costs compared to multi-stage processes, providing an option for the more economical production of iron oxide nanoparticles.
In the pulsation reactor, raw material mixtures are placed in a hot gas stream, being subjected to a calcination and material conversion via thermal shock treatment with extremely short residence times. [6,7] Due to the very high heating rate and the effective heat transfer, it is possible to affect the particle temperature, thus selectively influencing particle size, surface properties and phase composition. [8]
The special feature of our system is a pulsating hot gas stream, which enables a much more homogeneous temperature profile with narrower particle size distribution compared to the classical thermal process. [9] As a result, substances of definable properties can be produced. [10]
Results: Iron Oxide out of the Pulsation Reactor
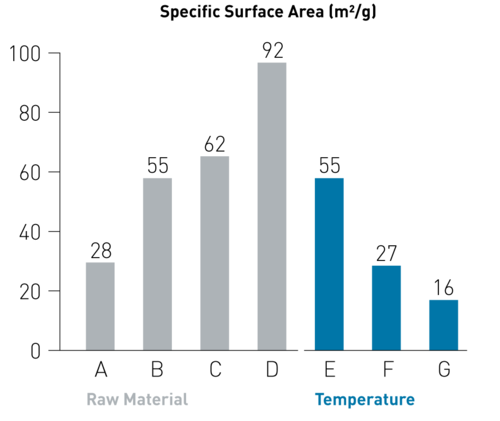
Variation of the process parameters in our pulsation reactor influences the specific surface area of the iron oxide nano particles (Figure 1), which directly correlates with the particle size, showing that the process can be used to produce high quality nano Fe2O3 powders with variable properties.
Figure 1. Specific surface area of nanoscale IBU-tec iron oxides, produced under different process conditions in the pulsation reactor.
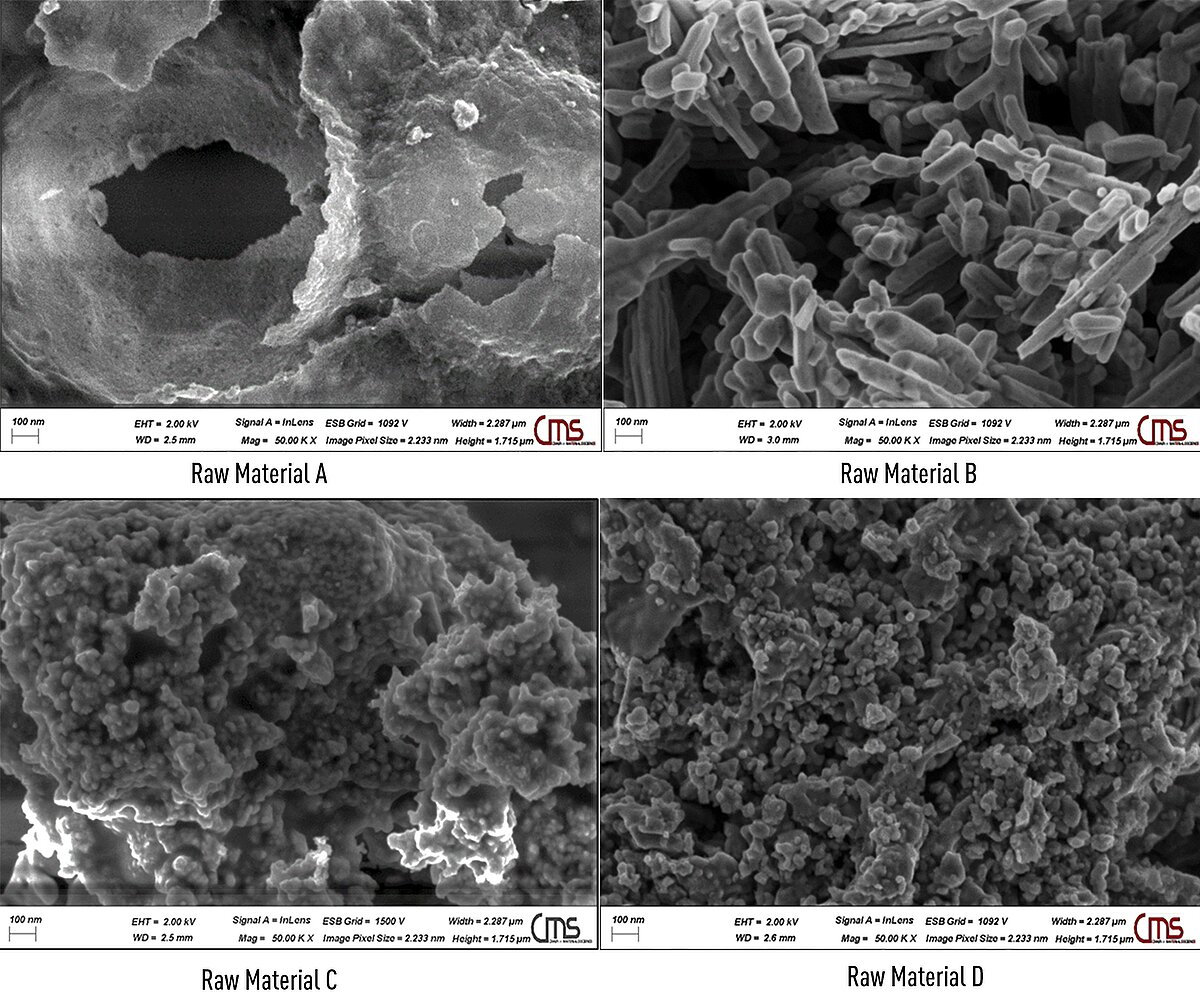
Electron microscopy (SEM) images show that the product consists of agglomerated nanoparticles with diameters between 30 and 40 nm, showing (SEM images, Figure 2) that the variation of the raw materials in the pulsation reactor results in iron oxide particles with different shapes and sizes. Thus, nano iron oxide particles with desired phase composition, particle shape and size can be developed to be directly utilized in an end-use application.
Figure 2: Scanning electron microscope images of the Fe2O3 products made in the pulsation reactor from various raw materials.
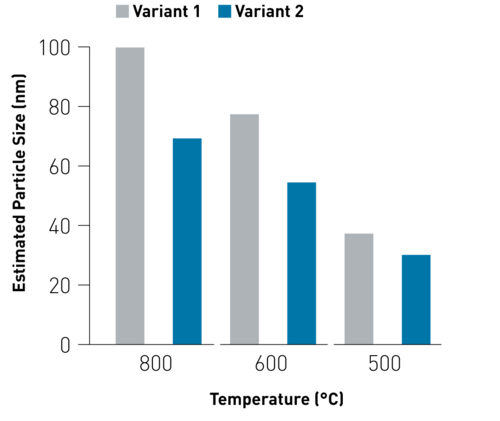
By varying process parameters, nano particles were produced in different particle sizes, which could be derived from the morphology examination, using SEM images (Figure 3).
Figure 3. Estimated particle sizes.
By transferring the obtained powders into suspensions with different solids contents, deagglomerating the particles and generating outputs for the targeted applications.
Conclusion
With the pulsation reactors, IBU-tec provides a worldwide exclusive thermal production process for fine-particle powders on a technological scale that is scalable to productions on the ton scale. Compared to processes such as sol-gel, chemical precipitation or wet chemical methods, iron oxide produced in the pulsation reactor has the advantage of the relative efficiency of a one stage process: A resource-saving production of nanoparticles with the desired phase composition, particle shape and size ready for end application use.
Sources
- M. Sartor, J. Schoppe, and T. Khalil, "Reduction of scale formation by coatings", SchmiedeJOURNAL2012, 2012.
- S.R. Narayanan, G.K. Surya Prakash, A. Manohar, Bo Yang, S. Malkhandi, und Andrew Kindler. Materials challenges and technical approaches for realizing inexpensive and robust iron–air batteries for large-scale energy storage. Solid State Ionics, 216:105–109, 2012.
- Albert Rössler, Georgios Skillas, and Sotiris E Pratsinis. Nanoparticles - Materials of the Future: Tailor-made materials. Chemistry in Our Time, 35(1):32–41, 2001
- IBU-tec. Financial News (DAGP): Major project in the growth area of battery materials for stationary energy storage with renowned partners. www.dgap.de/dgap/News/corporate/ibutec-advanced-materials-grosses-projekt-wachstumsbereich-batteriewerkstoffe-zur-stationaeren-energiespeicherung-mit-renommierten-partnern/, January 2019.
- Henning Weinrich, Jérémy Come, Hermann Tempel, Hans Kungl, Rüdiger-A Eichel, und Nina Balke. Understanding the nanoscale redox-behavior of iron-anodes for rechargeable iron-air batteries. Nano Energy, 41:706–716, 2017.
- Strobel, R.; Pratsinis, S. E. Flame Aerosol Synthesis of Smart Nanostructured Materials. J. Mater. Chem. 2007, 17 (45), 4743–4756. doi.org/10.1039/b711652g.
- Pratsinis, S. E. Flame Aerosol Synthesis of Ceramic Powders. Progress in Energy and Combustion Science. Elsevier Ltd January 1, 1998, pp 197–219. doi.org/10.1016/S0360-1285(97)00028-2.
- C. Hoffmann and M. Ommer: "Reaktoren für Fluid-Solid-Reaktionen: Pulsationsreaktoren", in Handbuch Chemische Reaktoren: Grundlagen und Anwendungen der Chemischen Reaktionstechnik, W. Reschetilowski, Hrsg., Berlin, Heidelberg: Springer Berlin Heidelberg, 2019, pp. 1-19 https://link.springer.com/referenceworkentry/10.1007/978-3-662-56444-8_50-1
- Stefan Ambrosius, Gerd Fischer, Tarek Khalil, and Lars Leidolph. DE 10 2006 046 805 A1: Process for the production of monooxides in the form of finely divided particles, 2007.
- Leidolph, L.; Khalil, T.; Dwuletzki, H.; Schoppe, J.; Quermann, R.; Sartor, M.; Wound, M.; Reichardt,T: "Use of nanoscale coating systems to reducehigh-temperaturecorrosion when reheating pre-material during open-die forging" BMBF final report, funding code: 03X0088 C, Düsseldorf 2012.